resultant wind - the vectorial average of all wind directions and speeds for a given level at a given place for a certain period, such as a month
Experiment Of Determine the Compotions of Air By MASS
- Determine Oxygen:
👉 You Can determine oxygen by using Copper
- Oxygen combine with copper heated to form Copper(III)Oxide
-Copper heated it will absorb oxygen
-Nitrogen is run to the globe
UZITO WAKE NI TOFAUTI NA WAKWANZA KWA SABABU YA KUONGEZEKA OXYGEN KWENYE COPPER
THIS IS MORE ACCURATE WAY OF DETERMINE OXYGEN THAN USE CANDLE
- Determine Nitrogen:
👉 You Can determine oxygen by using Mg
- Oxygen combine with copper heated to form Magnesium nitride
-Copper heated it will absorb oxygen
-Nitrogen is run to the globe
UZITO WAKE NI TOFAUTI NA WAKWANZA KWA SABABU YA KUONGEZEKA OXYGEN KWENYE MAGNESSIUM
Experiment Of Determine the Compotions of Air By VOLUME
- Determine Oxygen:
👉 You Can determine oxygen by using Candle
- Sodium hydroxide is used for absorb carbondiode from burning candle
- Oxygen iliyofunikiwa katika measuring cylinder ndiyo inayotumika kuwashia mshumaa
-Kiasi cha sodium hydroxide kitakacho panda kwenye cylinder ni tofauti na mwanzo hiyo ndo volume ya oxygen iloytumika kuwashia mshumaa , pia kumbua Carbondioxide ilinywa na sodium hydroxide
- Dissolved carbondioxide in sodium hydroxide make to raise volume of sodium hydroxide
-carbondioxide produced = oxygen used why Oygen used to make carbndioxide
Hii njia haileti Oxygen kwenye 21% kamili, kutokana kwa sababu
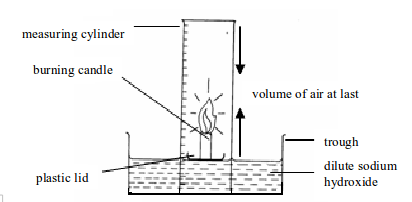
- Sio kiasi chote cha carbondioxide , kime-dissolve kwenye hydrogen chloride
- Baadhi ya Oxygen huwa haijatumika kwa kuwa mshumaa umewahi kuzima - Mshumaa unaweza kuzima kabla ya muda kutokana na accumulation of un-dissolved carbondioxe
- Oxygen hutumika kupunguza heat air kwenye cylinder - kwa sababu oxygen ni cool air

Experiment Of Determine the Compotions of Air -Oxygen By COMBUSTION
OBSERVATION
- Final Volume of the Air used is different to initial - Oxygen imetumika kwenye ku-burn copper
Carbondioxide
Determine the Presence of Carbondioxide By LIME WATER
Lime water is lemon juice
Water Vapour
Determine the Presence of Water Vapour By Deliquescent
Deliquescent Substances - absorb a large amount of water and be sufficiently soluble to dissolve in Eg: sukari
Hygroscopic Substances - substances which absorb moisture from air but do not change their state Eg: mchele
Ways Used to Determine Water Vapour
- Cobalt Chloride Paper- turn pink
- Anhydrous Cooper(III)Sulphate - turn hydrated blue crystal
Noble Gas
Polluntant
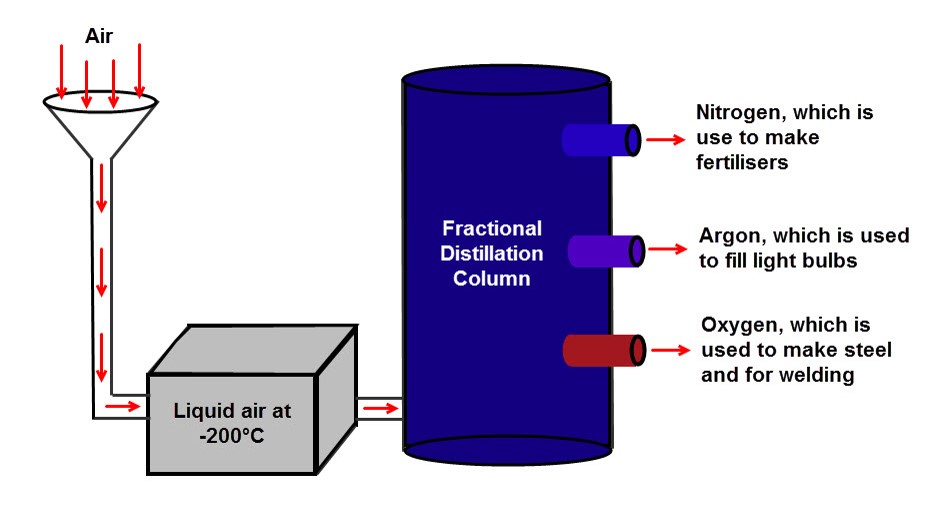
SEPARATION OF AIR
Make Oxygen From Air
Stages Of Air Separation
- Liquefying the air
-water vapour condensed
hutumia Nitrogen gas kupooza air
-carbon dioxide freezes at -79°C, and is removed.
Dry Ice is the common name for solid carbon dioxide (CO2). It gets this name because it does not melt into a liquid when heated; instead, it changes directly into a gas (a process known as sublimation).
-oxygen liquefies at -183°C.
-nitrogen liquefies at -196°C
- fractional distillation.
COMBUSTION
Combustible Material - Is any substance material burn
Supporter Of Combustion - is air or oxygen support combustion
Exothermic reactions are reactions or processes that release energy, usually in the form of heat or light.
Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect.
Oxygen is highly reactive gas
Reactive Gas - Support combustion
Non Reactive Gases (
Non-Reacting Gas
) - Also known as inert gases—are gases that do not undergo chemical reactions under specific conditions such as oxidization. These include argon, carbon dioxide, helium, and nitrogen.Why CO2 is not used for class a fire?
The carbon dioxide is also very cold as it comes out of the extinguisher, so it cools the fuel as well. CO2s may be ineffective at extinguishing Class A fires because they may not be able to displace enough oxygen to successfully put the fire out. Class A materials may also smolder and re-ignite.
Smolder - burn slowly with smoke but no flame.
Re-ignite - begin to burn again
Sodium hydroxide, also known as lye and caustic soda
Calcium chloride is an inorganic compound, a salt with the chemical formula CaCl2
/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
Comments
Post a Comment