He 👉 Atom , Pure element
H2 👉 Molecule , Pure Element
H2O 👉 Molecule , Compound
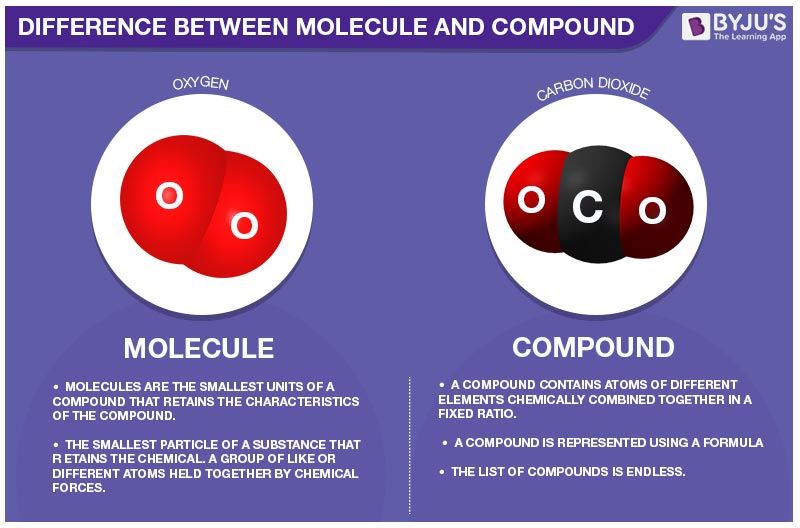
Molecule is made up of DIFFENT many atom.
Compound is made up of different atom
NOTE: ALL MOLECULE ARE NOT COMPOUND, ALL COMPOUND ARE MOLECULES
MORE
A molecule is a group of two or more atoms held together by chemical bonds. A compound is a substance which is formed by two or more different types of elements which are united chemically in a fixed proportion.
There is one Atom in Element
An atom is the part of an element. A particular element is composed of only one type of atom. Atoms are further composed of subatomic particles called electrons, protons and neutrons.
Atom is electrical neutral - contain same number of protons and electron
Ions - contain different number of protons and electron
Negative IONS - Anion
Positive IONS - Cation
There is :-
Ionic Compound
Covalent Compound | Molecular Compound
Ionic Compound - made up metal and 99% non metal Example NaCl = Na-Metal Cl-Non-Metal
Electron are not shared , are transfered electron. Example Contain positive and negative charge is
Na+
Cl-
Covalent Compound - made up of non metal Example H2O = H-Non-Metal O -Non-Metal
In Molecular Compound electron are shared between Atom.
Examples:
CO 👉 Molecular
Why C - Non-metal and O - Non-metal
MgCl2 👉 Ionic
Why Mg - Metal and Cl2 - Non-metal
SF6 👉 Ionic
Why S - Non-Metal and F6 - Non-metal
CaO 👉 Ionic
Why Ca - Metal and O - Non-metal
But be Careful
NH4 Cl 👉 Ionic
Why NH4+ - Non-Metal and Cl- - Non-metal
Ionic Compound are (usually) formed when a metal reacts with a nonmetal (or a polyatomic ion).
Comments
Post a Comment