Why do molecules at the surface of liquid have higher energy as compared to molecules in bulk?
When some liquid evaporates, the average speed of the molecules remaining will Answer
Substance is made up element and compound.
Element is the substance with limit of chemical analysis.
Chemical Analysis, the study of the chemical composition and structure of substances.
Compound is made up of two or more element.
Matter is made up of atoms, ions and molecules.
Matter increase in volume(expand) when temperature increase
Decrease in volume(contraction) when temperature decrease
Gas is easily compressible.
Liquid is slightly compressible
Solid is incompressible . Do not affected by the change of pressure.
Kinetic Theory of Matter
states that all matter is made of small particles that are in random motion and that have space between them.
The kinetic molecular theory of matter states
Matter is made up of particles that are constantly moving. All particles have energy, but the energy varies depending on the temperature the sample of matter is in. This in turn determines whether the substance exists in the solid, liquid, or gaseous state.
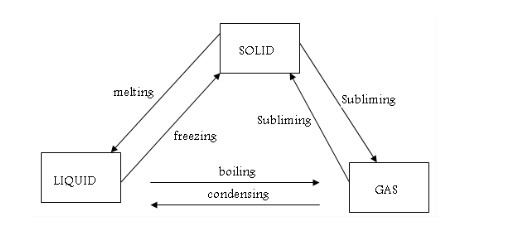
Melting
When solids are heated, their constituent particles (atoms, molecules or ions) get energy and vibrate more violently. Vibrations of these particles overcome (exceed) their binding forces. The particles become mobile. The crystalline structure of solid is destroyed. A liquid state is reached and the particles are free to move. The temperature at which this happens is called Melting Point
Melting point tell the strength of the force holding the particles.
Substances with high melting points have strong forces between their particles. Those with low melting points have weak forces between their particles.
Freezing
Is opposite of Melting.
The process is reversed at the same temperature if a liquid is cooled.
The melting point and freezing point of any given substance are both the same.
Melting is not affected by any changes in atmospheric pressure.
Boiling
liquid to vapour happen at particular temperature.
Evaporating
liquid to vapour happen at any temperature.
- takes place at the surface of liquid
The large surface area the faster liquid evaporates
The warmer the liquid is, the faster it evaporates. Thus, surface area and temperature affects the rate of evaporation of a liquid.
When a liquid is heated, its molecules get more energy and move faster .
As the heating goes on, its molecules vibrate even faster. Bubbles of gas (due to air dissolved in water)
Boiling Point
The molecules at the surface of the liquid gain enough energy to overcome the forces holding them together. They break away from the liquid and from a gas (vapour).
The temperature at which a liquid boils explains how strong the forces holding its particles (molecules) together are.
Formation Of Rain
The particles of matter are held together by the strong electrostatic force
Solid state
Solid made up of small particles tha are closer together
No free movement of particles .
They can not move around freely , instead vibrate about fixed position.
Make solid to have fixed shape.
Liquid State
force of attraction is weaker than solid.
Liquids particles have more kinetic energy than solids. and can move along each other .
The binding forces are strong when particles come close to one another.
It is thought that the particles of a liquid are fairly randomly arranged but consist of "clusters" closely packed together. This property makes a liquid to have a definite volume.
However, since the particles are fairly free to move a liquid does not have any characteristic shape
liquid will always take the shape of its container.
Liquids have more kinetic energy than solids. If you add heat energy to a liquid, the particles will move faster around each other as their kinetic energy increases. Some of these particles will have enough kinetic energy to break their liquid bonds and escape as a gas (evaporation).
Gaseous State
Particles moves independently.
Particles are far apart.
Its particles have more energy than solid and liquid
Particles move rapidly and randomly - in container when move collide with each.
Physical Change
only physical change but nature not changed
Characteristics of Physical Change
- No new substance formed
- no change of weight
- Changed Back
- need little heat to be change
Note : Change Physical Properties
Chemical Change
refers to a change which is permanent in nature
eg : wood to ash
Characteristics of Physical Change
- new substance formed
- change of weight
- not Changed Back
- need more heat to be change
Note : Change Chemical Properties
Napthalene is the ingradient found in mothballs
https://www.youtube.com/watch?v=MostFAuz02o
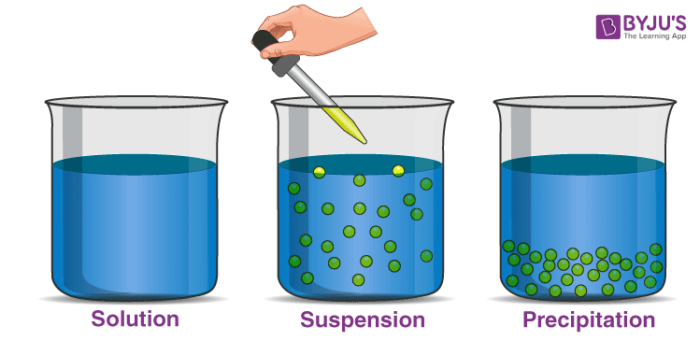
Suspension is a heterogeneous mixture of a finely distributed solid in a liquid.
Eg; Muddy water.


Comments
Post a Comment