What is zinc granules? Explain with examples?
- It is a single replacement reaction where zinc metal displaces hydrogen to form hydrogen gas & zinc chloride, a salt.
- Zinc granules are the solid crystals of zinc.
- Zinc reacts easily with the acid to produce hydrogen bubbles.
- Zn + HCl → H2 + ZnCl2 is the reaction involving zinc and hydrochloric acid.
ZINC GRANULES
BOSCH PROCESS
Reactions of metals with water
- When a metal reacts with water, a metal hydroxide and hydrogen are formed.
- For example:
- sodium + water ➞ sodium hydroxide + hydrogen.
- calcium + water ➞ calcium hydroxide + hydrogen.
STEAM MEATHANE (STEAM REFORMING) hydrocarbon + steam
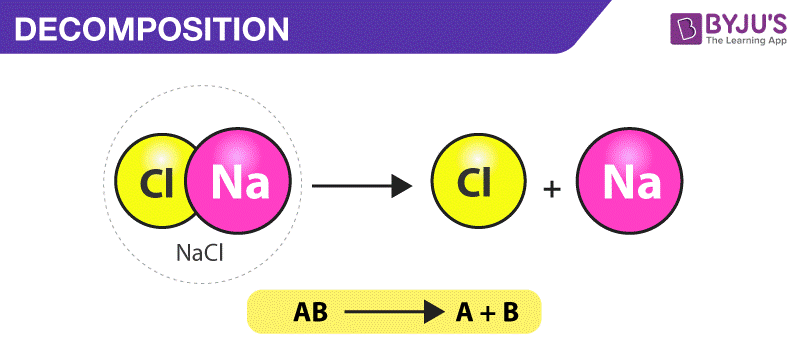
Chemical decomposition, or chemical breakdown, is the process or effect of simplifying a single chemical entity (normal molecule, reaction intermediate, etc.) into two or more fragments. ... In short, the chemical reaction in which two or more products are formed from a single reactant is called a decomposition reaction.
anhydrous
(of a substance, especially a crystalline compound) containing no water.
Chemical decomposition, or chemical breakdown
The hydrogen mixes with oxygen in the air forming an explosive mixture.
Hydrogen is very flammable
Hydrogen gas is inert towards most common drying agents, so you could try using a drying tube filled with anhydrous salts like CaCl2, Na2SO4, MgSO4 or you can bubble it through cc. H2SO4.


Comments
Post a Comment