Ionic Bond(electrovalent bond) : are elelectron transfered from one element to another. Its happen when metal and non metal are reacted , - they transferd electrons
Covalent Bond : electron are shared. Happen when similar atom react (Non- metal and Non-metal are reacted) - they shared electrons
Properties Of Halogen
- They have very high electronegativities.
- They have seven valence electrons (one short of a stable octet).
- They are highly reactive, especially with alkali metals and alkaline earths. ...
- Because they are so reactive, elemental halogens are toxic and potentially lethal.
Difference between valency and oxidation number is that
Valency is the maximum number of electrons an atom can lose, gain or share to become stable,
Oxidation number is the number of electrons an atom can lose or gain to form a bond with another atom.
There is strong correlation(Kuna uwiano mkubwa )
Why is oxidation number negative?
Why there is negative oxidation number - If the oxidation number is positive, then this means that the atom loses electrons, and if it is negative, it means the atom gains electrons. If it is zero, then the atom neither gains nor loses electrons. ... If an atom gains electrons, its oxidation number is negative, so we can say that the atom undergoes reduction.
Redox is a type of chemical reaction in which the oxidation states of atoms are changed.
Oxidation is a reaction that removes an electron from a substance, reduction is a reaction that adds electrons to a substance.
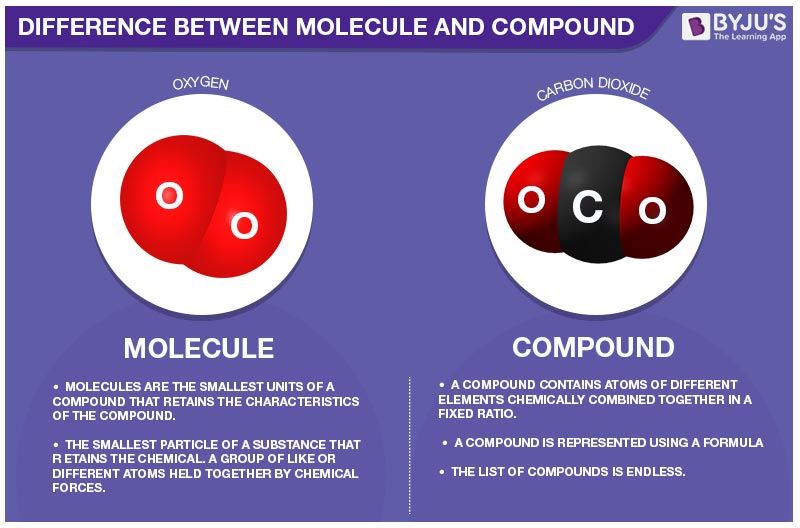
A molecule is a group of two or more atoms held together by chemical bonds. A compound is a substance which is formed by two or more different types of elements which are united chemically in a fixed proportion. ... An example of a molecule is ozone. An example of a compound is table salt (sodium chloride).
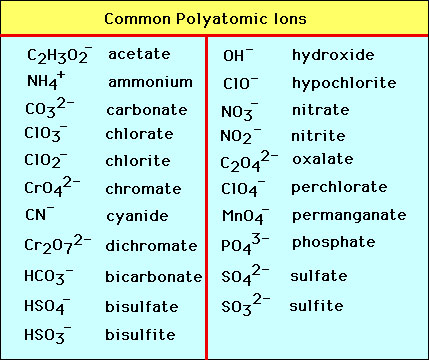
Polyatomic ion( Molecular ion )


Comments
Post a Comment